Metal-binding peptides
Design of Lead-Binding Peptides
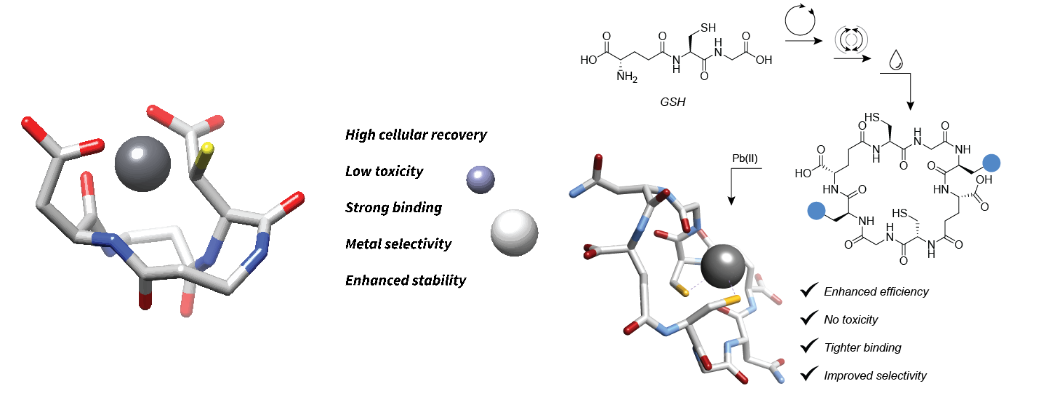
In a joint project(s) with the group of Dr. Michal Shoshan (Univ. Zürich, Switzerland), we focused on the design of selective lead-binding peptides.1-3 On the computational side, we capitalized on our previous work, which provided solid and calibrated computational protocols based on the DFT-D3//COSMO-RS method, for estimating stability constants of various [M2+:peptide/amino acid] complexes, as well as on recently developed protocols for exhaustive and efficient conformational sampling of peptides and macrocycles. Therefore, we were able to provide clear computational evidence concerning the binding modes of Pb2+ in complexes with newly designed cyclic tetrapeptides, with quantitative agreement between the computed and experimental stability (dissociation) constants.1,2 This enabled us to explain the selectivity of the tetrapeptides for Pb2+ over Ca2+ and Zn2+. A similar methodology was used to design, by joint computational and experimental efforts, novel cyclic octapeptides composed of two glutathione units for lead binding.3 These were shown to outperform the corresponding monomers and are promising candidates for lead detoxification/scavenging from biological materials.
References:
1. Mohammed, T. A.; Meier, C. M.; Kalvoda, T.; Kalt, M. A.; Rulíšek, L.; Shoshan, M. S.: Potent Cyclic Tetrapeptides for Lead Detoxification. Angew. Chem. Int. Ed. 2021, 60, 12381‑12385. https://doi.org/10.1002/anie.202103217
2. Sauser, L.; Mohammed, T. A.; Kalvoda, T.; Feng, S.‑J.; Spingler, B.; Rulíšek, L.; Shoshan, M. S.: Thiolation and Carboxylation of Glutathione Synergistically Enhance Its Lead-Detoxification Capabilities. Inorg. Chem. 2021, 60, 18620–18624. https://doi.org/10.1021/acs.inorgchem.1c03030
3. Sauser, L.; Kalvoda, T.; Kavas, A.; Rulíšek, L.; Shoshan, M. S.: Cyclic Octapeptides Composed of Two Glutathione Units Outperform the Monomer in Lead Detoxification. ChemMedChem 2022, 17, e202200152. https://dx.doi.org/10.1002/cmdc.202200152

