Metalloenzymatic reactions
To understand all details of metalloenzymatic reactions, enormous efforts, both experimental and theoretical, have been exerted over the last decades. While experiments (e.g., X-ray crystallography, various spectroscopic techniques, electrochemistry) are crucial in initial phases of our understanding of a particular system, theoretical calculations complement these data by providing a unique one-to-one structure-energy mapping. Our efforts in the past years focused on two coupled binuclear systems: tyrosinase with a dicopper catalytic core,3-7 detailed below, and non-heme diiron (NHFe2) Δ9-desaturase.1,2
Reaction mechanism of tyrosinase (Ty), a metalloenzyme featuring coupled binuclear copper active site
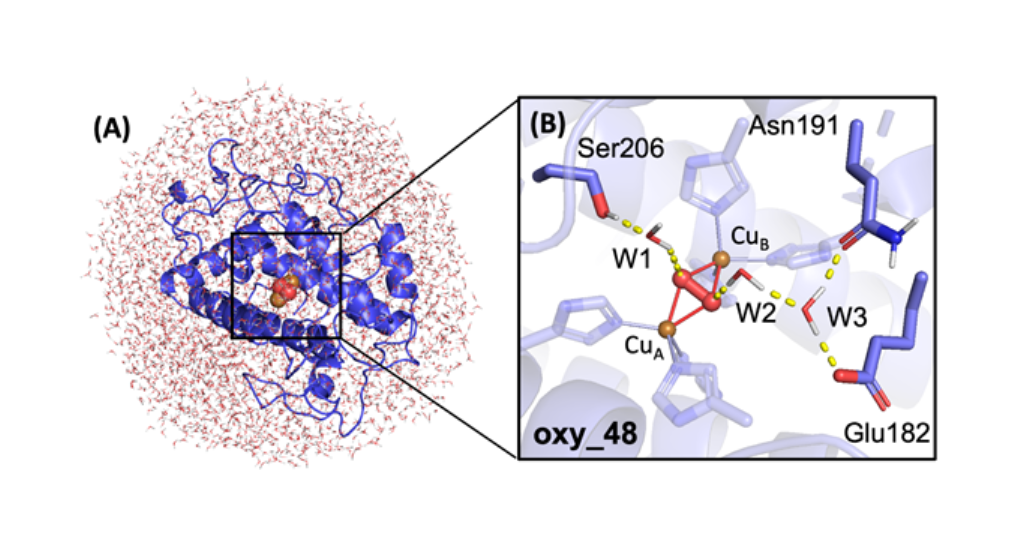
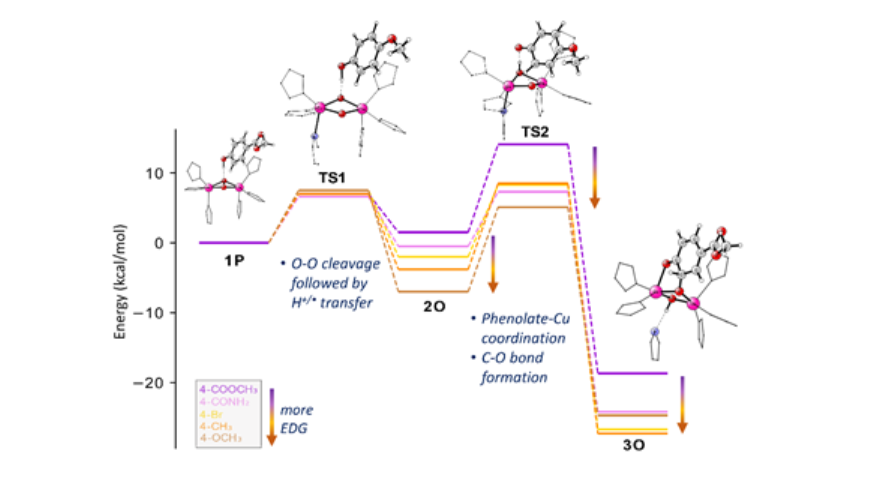
In the next step, by correlation of exhaustive QM/MM and QM-cluster modeling with kinetic and spectroscopic experiments, we clearly identified and characterized the elusive ternary catalytic intermediate (Ty+O2+substrate).5 This allowed us to explore the monooxygenation with methyl 4-hydroxybenzoate, and to validate the revealed mechanism with different para-substituent monophenols, showing an interesting reversal in the RLS of the reaction.6
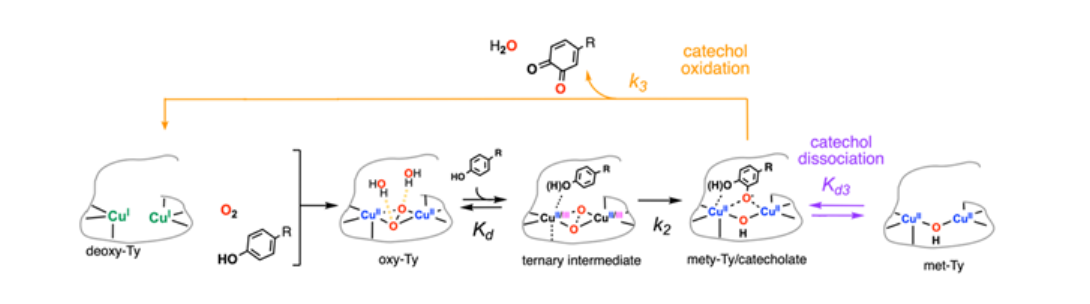
Ongoing efforts are focused on catechol oxidation by Ty which is the final step in the full Ty catalytic cycle. In the future, we will study the other members of the coupled binuclear copper (CBC) family - catechol oxidase and o-aminophenol oxidase, to understand the varying chemoselectivity across the CBC family.7
References:
1. Bím, D.; Chalupský, J.; Culka, M.; Solomon, E. I.; Rulíšek, L.; Srnec, M.: Proton-Electron Transfer to the Active Site Is Essential for the Reaction Mechanism of Soluble Δ9-Desaturase. J. Am. Chem. Soc. 2020, 142, 10412−10423. https://doi.org/10.1021/jacs.0c01786
2. Tupec, M.; Culka, M.; Machara, A.; Macháček, S.; Bím, D.; Svatoš, A.; Rulíšek, L.; Pichová, I.: Understanding desaturation/hydroxylation activity of castor stearoyl Δ9-desaturase through rational mutagenesis. Comput. Struct. Biotechnol. J. 2022, 20, 1378–1388. https://doi.org/10.1016/j.csbj.2022.03.010
3. Stańczak, A.; Chalupský, J.; Rulíšek, L.; Straka, M.: Comprehensive Theoretical View of the [Cu2O2] Side-on-Peroxo-/Bis-μ-Oxo Equilibria. ChemPhysChem 2022, 23, e202200076. https://doi.org/10.1002/cphc.202200076
4. Kipouros, I.; Stańczak, A.; Culka, M.; Andris, E.; Machonkin, T. R.; Rulíšek, L.; Solomon. E. I.: Evidence for H-bonding interactions to the m-h2:h2-peroxide of oxy-tyrosinase that activate its coupled binuclear copper site. Chem. Commun. 2022, 58, 3913‑3916. https://doi.org/10.1039/D2CC00750A
5. Kipouros, I.; Stańczak, A.; Ginsbach, J. W.; Andrikopoulos, P. C.; Rulíšek, L.; Solomon, E. I.: Elucidation of the tyrosinase/O2/monophenol ternary intermediate that dictates the monooxygenation mechanism in melanin biosynthesis. Proc. Natl. Acad. Sci. 2022, 119, e2205619119. https://doi.org/10.1073/pnas.2205619119
6. Kipouros, B. I.; Stańczak, A.; Dunietz, E. M.; Ginsbach, J. W.; Srnec, M.; Rulíšek, L.; Solomon, E. I. Experimental Evidence and Mechanistic Description of the Phenolic H-Transfer to the Cu₂O₂ Active Site of oxy-Tyrosinase. J. Am. Chem. Soc. 2023, 145 (38), 22866–22870. https://doi.org/10.1021/jacs.3c07450
7. Stańczak, A.; Kipouros, I.; Eminger, P.; Dunietz, E. M.; Solomon, E. I.; Rulíšek, L., Coupled Binuclear Copper Sites in Biology: An Experimentally-Calibrated Computational Perspective. Coord. Chem. Rev. 2025, 525, 216301. https://doi.org/10.1016/j.ccr.2024.216301

