Protein-ligand interactions
STING
STING (STimulator of INterferon Genes) is a key protein in the immune system and a prime medical target. Collaborative efforts of synthetic and computational chemists and biologists led by Dr. Gabriel Birkuš (STING Team@IOCB) resulted in several potent compounds that were patented and are now offered for licensing via the IOCB Tech. Computational efforts within the STING project provided a deep understanding of physico-chemical principles related to the binding of cyclic dinucleotides (CDNs) to the STING, such as an interplay of entropy and enthalpy, dynamical aspects of binding, and conformational behavior of CDNs. Computations also suggested specific mutations in the STING protein that can influence the binding of CDNs and thus highlight the role of various types of interactions in the [STING:CDN] complexes. Three medicinal chemistry (theoretical and experimental) studies were also published to assess and explain the affinity of the selected novel CDNs for STING.
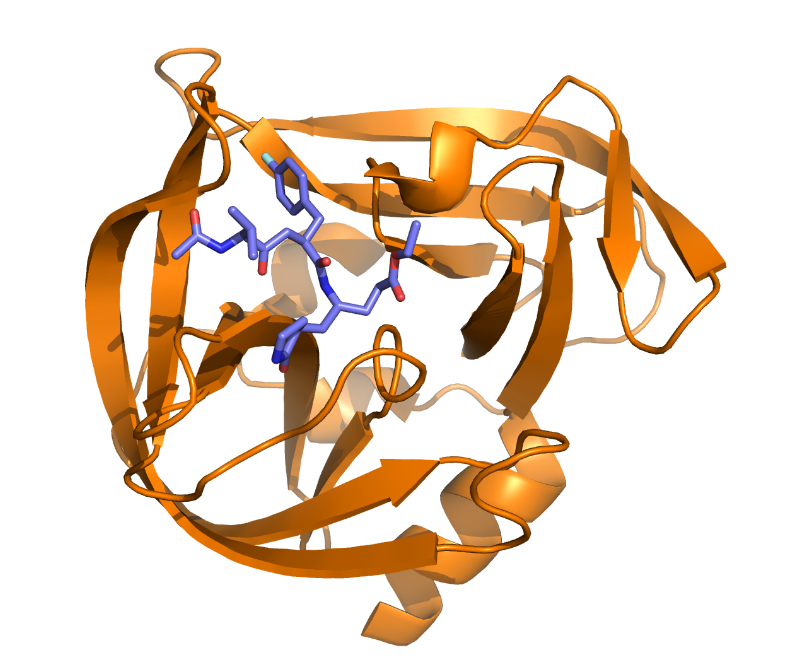
HRV 3C Protease
Human Rhinovirus is the cause of the common cold in humans. Its genetic information is translated into a long polyprotein, which is then mostly processed by the 3C Protease (HRV 3C) in order to create viral proteins. When the activity of this protease is inhibited or halted, the viral replication is severely impacted. This protease has a chymotrypsin-like fold but features a cysteine as an active residue, which makes it a great target for drug design. With the group of Dr. Gabriel Birkuš, we are working on developing drugs targeting this protease. For the newly designed drugs, computational predictions using methods of quantum chemistry, molecular dynamics or semi-empirical computational methods can aid in prioritizing promising therapeutic agents for laboratory synthesis and testing. Understanding key interactions between the therapeutic agents and the protein can also help with the design of new potent inhibitors of this protease.