Exotic chemical bonds
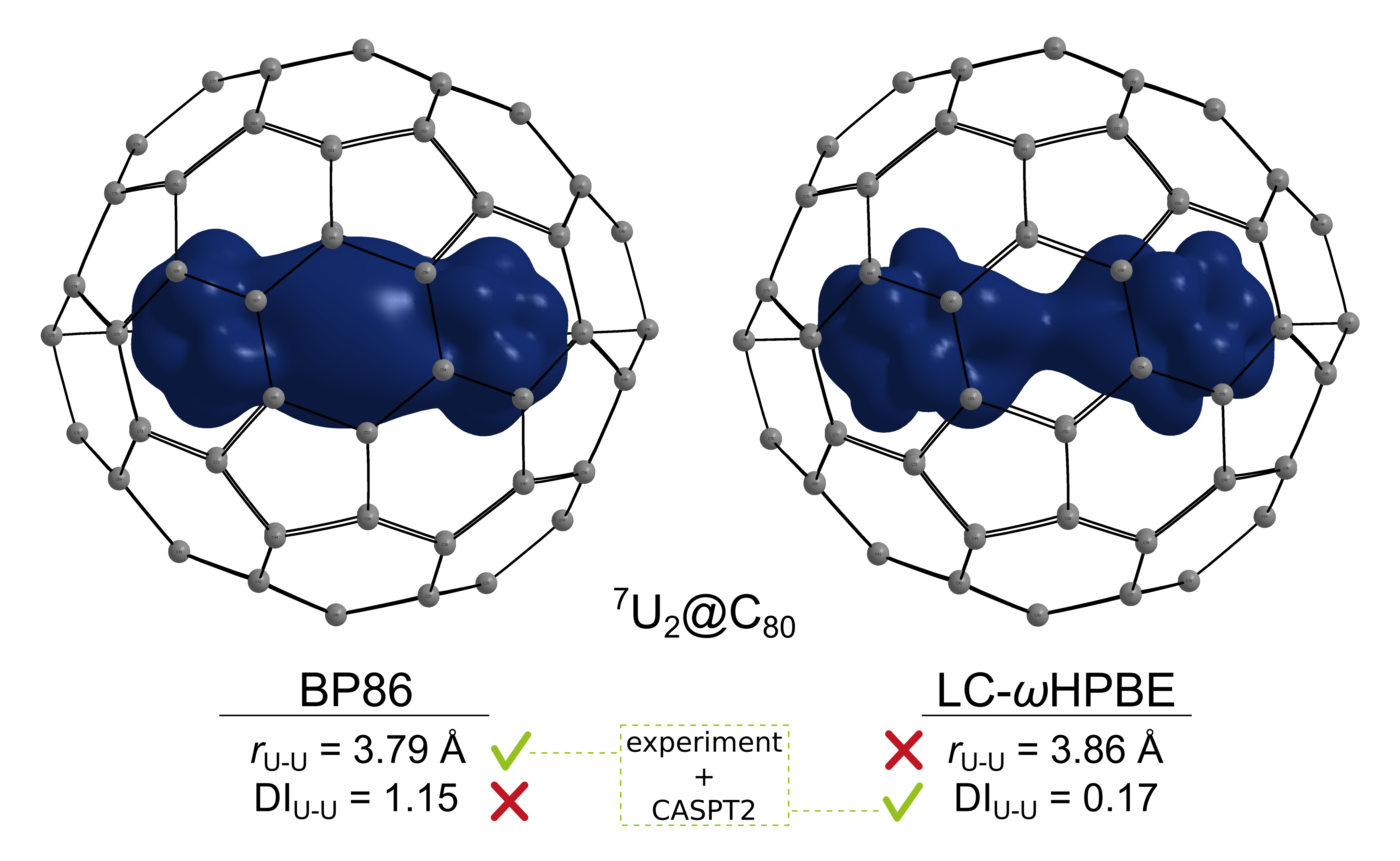
Actinide-actinide bonds in fullerenes
Actinide–actinide bonds are very rare. So far the An–An bonds have been only described in a gas-phase U2 and (indirectly) Th2 diatomics, and in the interior of endohedral fullerenes, where a strong metal-cage interaction keeps the two actinide atoms at distances where An–An bonds are formed. In 2015, we predicted actinide-actinide bonding in U2@Ih(7)-C80, coined unwilling bonding.1 This molecule was later confirmed experimentally.2 In 2020, we searched for actinide-actinide bonding in An2@D5h(1)-C70, An2@Ih(7)-C80, and An2@D5h(1)-C90 (An=Ac-Cm) series of endohedral metallofullerenes (EMFs). We showed that most of the studied An2@C70 and An2@C80 systems feature one or more one-electron two-center actinide-actinide bonds, in particular in Th and Pa EMFs.3 Notably, Th2@C80 predicted therein was later characterized experimentally.4 However, our benchmark study for theoretical predictions of actinide-actinide bonding revealed, that the DFT BP86-predicted bonding is overestimated.5 High-level ab initio calculations confirmed bonding in Th and Pa systems and in U2@C60, but only minor interaction was found in U2@C80 and heavy-actinide (Np, Pu, Am, Cm) compounds.
Fullerene molecular crystal
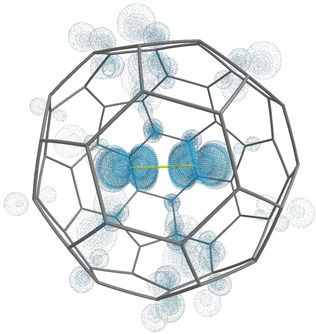
An interesting example of exotic bonding in the endohedral fullerenes is molecular crystal F2-@C60+. In collaboration with G. Frenking (University of Marburg), C. Foroutan-Nejad (Polish Academy of Sciences), and I. Fernandez (Universidad Complutense of Madrid) we have predicted that F2 molecule enclosed in C60 oxidizes the fullerene cage and stabilizes itself as F2- ion.6 Notably, the interaction between F2- and C60+ is predicted to be of purely electrostatic nature, making this molecule a single-molecule crystal. Additionally, F2-@C60+ is a rare example of an endohedral fullerene, where the enclosed moiety is negatively charged while the fullerene is oxidized.
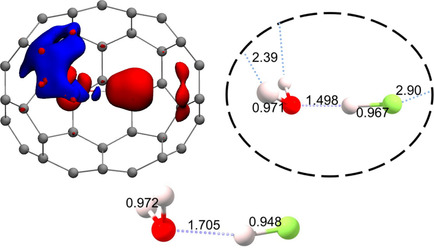
Charge-depletion bonding inside fullerenes
References:
1. Foroutan-Nejad, C.; Vícha, J.; Marek, R.; Patzschke, M.; Straka, M. Unwilling U–U Bonding in U2@C80: Cage-Driven Metal–Metal Bonds in Di-Uranium Fullerenes. Phys. Chem. Chem. Phys. 2015, 17, 24182–24192. https://doi.org/10.1039/C5CP04280A
2. Zhang, X.; Wang, Y.; Zhang, J.; Wang, C.; Wang, Y.; Wang, S.; Wang, G.; Wang, B. U2@Ih(7)-C80: Crystallographic Characterization of a Long-Sought Fullerene Cage with a U–U Bond. J. Am. Chem. Soc. 2017, 139, 16447–16450. https://doi.org/10.1021/jacs.7b10865
3. Jaroš, A.; Foroutan-Nejad, C.; Straka, M. From π Bonds without σ Bonds to the Longest Metal–Metal Bond Ever: A Survey on Actinide–Actinide Bonding in Fullerenes. Inorg. Chem. 2020, 59, 12608–12615. https://doi.org/10.1021/acs.inorgchem.0c01713
4. Zhuang, J.; Morales-Martínez, R.; Zhang, J.; Wang, Y.; Yao, Y.-R.; Pei, C.; Rodríguez-Fortea, A.; Wang, S.; Echegoyen, L.; de Graaf, C.; Poblet, J. M.; Chen, N. Characterization of a Strong Covalent Th3+–Th3+ Bond Inside an Ih(7)-C80 Fullerene Cage. Nat. Commun. 2021, 12, 2372. https://doi.org/10.1038/s41467-021-22659-2
5. Jaroš, A.; Straka, M. Unraveling Actinide–Actinide Bonding in Fullerene Cages: A DFT versus ab Initio Methodological Study. Phys. Chem. Chem. Phys. 2023, 25, 31500–31513. https://doi.org/10.1039/D3CP03606E
6. Zhang, J.; Wang, Y.; Wang, S.; Wang, G.; Wang, B. Buckyball Difluoride F2-@C60+—A Single-Molecule Crystal. Angew. Chem. Int. Ed. 2018, 57, 13931–13934. https://doi.org/10.1002/anie.201809699
7. Zhou, X.; Zhang, J.; Wang, Y.; Wang, S.; Wang, G.; Wang, B. Isolation of the Simplest Hydrated Acid. Sci. Adv. 2017, 3, e1602833. https://doi.org/10.1126/sciadv.1602833
8. Jaroš, A.; Badri, Z.; Bora, P. L.; Bonab, E.; Marek, R.; Straka, M; Foroutan-Nejad, C. How Does a Container Affect Acidity of Its Content: Charge Distribution and Acidity of H2O@C60. Chem. Eur. J. 2018, 24, 4245–4249. https://doi.org/10.1002/chem.201706017

