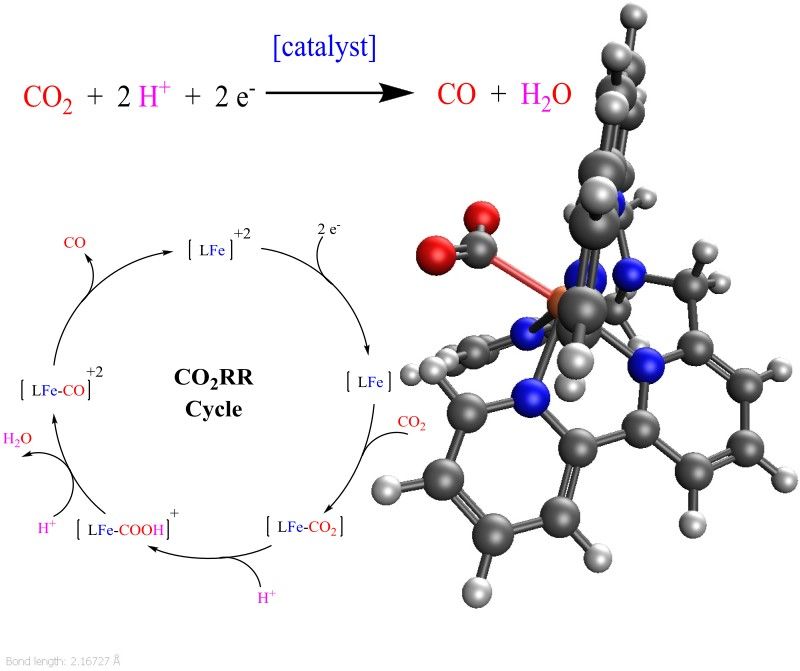
CO2 reduction
Rising global temperatures pose severe risks for modern society. This rise is mainly caused by the greenhouse effect of CO2 anthropogenically released into the atmosphere. Measures for the capture of CO2 suffer greatly from the limited supply of profitable uses for stored CO2 and the resulting lack of economical demand for it. If mankind were able to cost-efficiently mimic photosynthesis to turn CO2 into a spectrum of hydrocarbon-based products, this problem would vanish. On the other hand, procedures that allow the synthesis of the entire spectrum of petrochemical products from CO and H2 have been known, refined, and implemented for over a century. Our research focuses on the development of catalysts for the efficient conversion of CO2 into CO. In close collaboration with experimental scientists in the field of artificial photosynthesis, we use state-of-the-art quantum chemical tools to predict optimal reaction conditions for and chemical features of potential catalysts. Our recent contribution is depicted in the figure below: computations helped us to obtain essential details of the CO2RR catalyzed by the depicted hepta-coordinate iron-polypyridine complex and fully characterized individual reaction steps.